ethyne lewis structure|C2H2 Lewis Structure, Geometry : Tuguegarao The chemical formula of ethyne is C2H2. It consists of two carbon atoms and two hydrogen atoms. Ethyne is a colorless gas with a distinct odor and is highly flammable. The C2H2 .
EZ2 lotto results, March 2022. Philippine Charity Sweepstakes Office (PCSO), Philippines. Monthly results summary and history.Dengan bergabung bersama dengan winbet 299, anda tidak perlu lagi khawatir karena akun anda bisa digunakan untuk bermain semua permainan yang sudah disebutkan di atas. Untuk itu, segera bergabung dan nikmati kemenangan tiada henti bersama dengan WINBET299. Bonus Menarik Bergabung dan Deposit Setiap Hari. Bonus Member Baru
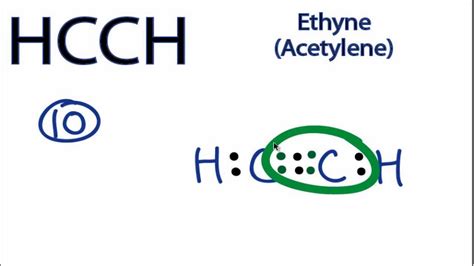
ethyne lewis structure,In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Ethyne or Acetylene. Note that we'll need a triple bond between the Carbon atoms for.In drawing the Lewis structure for C 2 H 2 (also called ethyne) you'll find that you don't have enough valence electrons available to satisfy the octet for each element (if you use . In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Acetylene (Ethyne). ---- Steps to Write Lewis Structure .
C2H2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms. There are no lone pairs on carbon or hydrogen . Lewis Dot Structure of C2H2 or CHCH (Acetylene or ethyne) kentchemistry.com. 25.1K subscribers. Subscribed. 299. 176K views 12 years ago Every Video. I quickly take you through how to draw.The chemical formula of ethyne is C2H2. It consists of two carbon atoms and two hydrogen atoms. Ethyne is a colorless gas with a distinct odor and is highly flammable. The C2H2 .
A key component of using Valence Bond Theory correctly is being able to use the Lewis dot diagram correctly. Ethene has a double bond between the carbons and single bonds .ethyne lewis structure C2H2 Lewis Structure, Geometry Ethyne, or acetylene, is the simplest alkyne with a carbon-carbon triple bond. Learn how ethyne is prepared, what its molecular shape and bond lengths are, and how it is used in welding, lighting, and polymerization.The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds. Experimentally, the four carbon . A step-by-step explanation of how to draw the HCCH Lewis Dot Structure (Ethyne).For the HCCH structure use the periodic table to find the total number of val.
H. 4. ) Lewis Structure, Hybridization. Ethene's lewis structure can be built by VSEPR rule. Most stable structure is taken as the lewis structure of ethene. Hybridization of atoms in ethene molecue can be found from lewis structure. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial.Drawing the Lewis Structure for HCCH. HCCH (Ethyne) can also be written as C 2 H 2. Ethyne is also called Acetylene. For the HCCH Lewis structure you'll need to form a triple bond between the two carbon atoms. Hydrogen atoms only need two electrons for a full outer shell. There are a total of 10 valence electrons for the HCCH Lewis structure.
C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle. C2H2 is a chemical formula for Ethyne, a gaseous alkyne hydrocarbon. It has been used widely for welding and cutting purposes. This molecule is also known by the name Acetylene. The compound has a simple structure and is made up of two carbon atoms .
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”. Ethene or C2H4 is a common straight-chain acyclic alkene and an important member of organic hydrocarbons. Having a double C=C bond, it is unsaturated and this gives rise to several properties. Here, we learned about how to draw the proper Lewis Structure and find out the molecular geometry of an ethylene molecule.
C2H2 Lewis Structure, Geometry Ethene or C2H4 is a common straight-chain acyclic alkene and an important member of organic hydrocarbons. Having a double C=C bond, it is unsaturated and this gives rise to several properties. Here, we learned about how to draw the proper Lewis Structure and find out the molecular geometry of an ethylene molecule. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene).For the C2H4 structure use the periodic table to find the total number of val. An orbital view of the bonding in ethyne. Ethyne is built from hydrogen atoms (1s 1) and carbon atoms (1s 2 2s 2 2p x 1 2p y 1).The carbon atom does not have enough unpaired electrons to form four bonds (1 to the hydrogen and three to the other carbon), so it needs to promote one of the 2s 2 pair into the empty 2p z orbital. This is . Ethene is the formal IUPAC name for H 2 C=CH 2, but it also goes by a common name: Ethylene. The name Ethylene is used because it is like an ethyl group ( CH2CH3 C H 2 C H 3) but there is a double bond between the two carbon atoms in it. Ethene has the formula C2H4 C 2 H 4 and is the simplest alkene because it has the .

Lewis Dot of Ethyne (Acetylene) C 2 H 2. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Acetylene is an unsaturated hydrocarbon with a triple bond.
ethyne lewis structureLewis Dot of Ethyne (Acetylene) C 2 H 2. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Acetylene is an unsaturated hydrocarbon with a triple bond.
The correct Lewis structure for ethene is shown below: In the molecule ethene, both carbon atoms will be sp2 hybridized and have one unpaired electron in a non-hybridized p orbital. These p-orbitals will undergo .

Lewis Structure of Acetylene (C2H2) Lewis Structure is the pictorial representation showing how the valence electrons are participating in bond formation. To study this, first, it is crucial to know the electronic .Ethyne, also known as acetylene, is an organic chemical compound with the chemical formula C2H2. Since the entire chemical composition only features hydrogen and carbon atoms, this compound is a hydrocarbon. .The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms .
Step 3: Connect each atoms by putting an electron pair between them. Now in the C2H2 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-hydrogen atoms. This indicates that these atoms are chemically bonded with each other in a C2H2 molecule. Step 4: Make the outer atoms stable.Drawing the Lewis Structure for C 2 H 2 - Ethyne or Acetylene. Viewing Notes: With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms.; Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure. Note that Hydrogen only needs two valence electrons .
A. Chemical formula of Ethyne. The chemical formula of ethyne is C2H2. It consists of two carbon atoms and two hydrogen atoms. Ethyne is a colorless gas with a distinct odor and is highly flammable. The C2H2 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. II.
October 31, 2023 by Deep. The information on this page is fact-checked. C 2 H 2 Lewis structure. C 2 H 2 (acetylene or ethyne) has two carbon atoms and two hydrogen atoms. In the C 2 H 2 Lewis structure, there is a triple bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair.
ethyne lewis structure|C2H2 Lewis Structure, Geometry
PH0 · Lewis Structure for C2H2 (Ethyne)
PH1 · Lewis Dot Structure of C2H2 or CHCH (Acetylene or ethyne)
PH2 · How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)
PH3 · Ethyne (Acetylene)
PH4 · C2H2 Lewis Structure, Geometry
PH5 · C2H2 Lewis Structure Tutorial
PH6 · C2H2 (Ethyne) Lewis structure
PH7 · C2H2 (Acetylene
PH8 · 1.8: sp² Hybrid Orbitals and the Structure of Ethylene
PH9 · 1.13: Ethane, Ethylene, and Acetylene